Homemade soap is a Must about DIY project! It can be of different types depending on their use. So we will have a cleanser, kitchen soap, personal soap, laundry soap, and so on. Today I want to focus on bar soap for personal usage, but first of all, let’s go deeper and see what soap is. This post is an excerpt from Soap making 101 with the cold method, have a look at it by clicking here. You will find all the information to master this hobby.
What is soap??
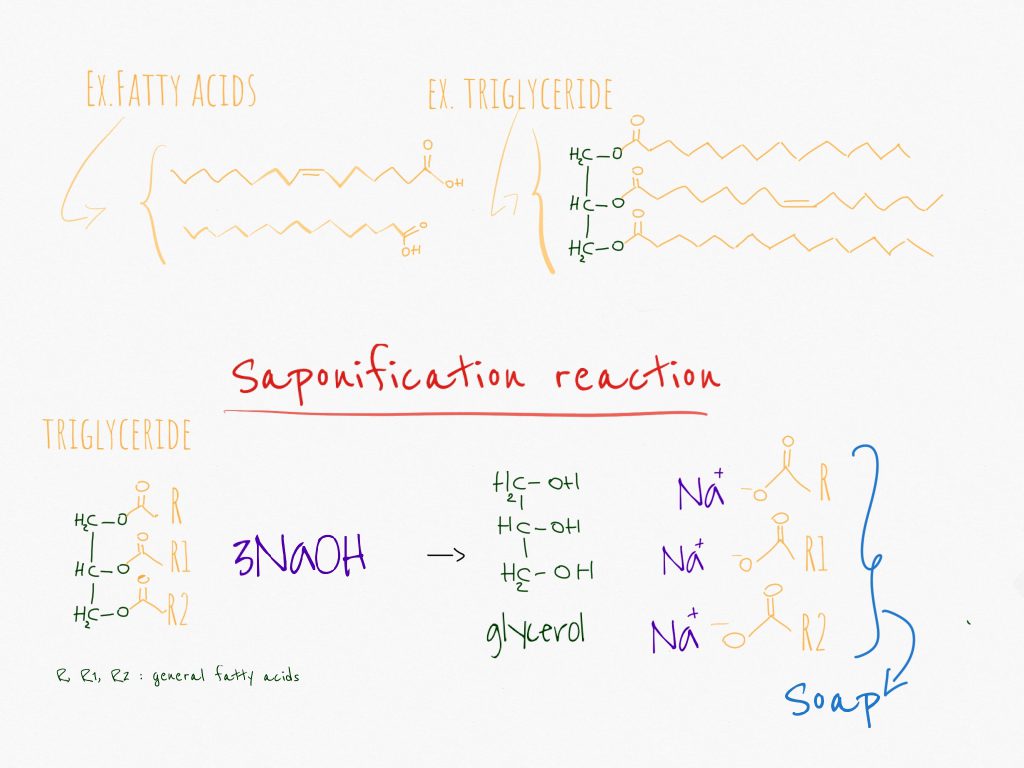
In chemistry, soaps are defined as salts of fatty acids. They are obtained from oils and fats that are made of fatty acids and triglycerides. The reaction of these components with an aqueous solution of NaOH or KOH is called saponification and produces soap and glycerol (also called glycerine).
The chemical structure of the soap presents two distinct parts an apolar tail and a polar head. This dual nature makes the soap stable not only in the aqueous solution but also in the drops of fat. The apolar tail will attack the dirty surface on our skin while the polar head will bring them to the aqueous solution, removing at the end the dirty from our skin. This concept was already explained in my previous post (–>take a look here) where I wrote about the emulsifiers.
Learn everything about soap making with my comprehensive course! Everything from ingredients, to lye discount, superfat, and much more!
NB: HANDLE CAREFULLY the solution of NaOH (Sodium hydroxide, also known as caustic soda and lye). This compound is sold as white crystals. It’s a corrosive product and in contact with water produces heat that could ignite combustible materials. During the preparation of your own soap ALWAYS wear protective goggles and gloves (natural rubber, PVC, or nitrile)!! First, think about your safety!
After these recommendations, it seems complicated and dangerous but it’s not if some precautions are taken. The chemist inside me wants to do soon again a soap.. shortly I will report each step for its realization! 🙂
Ready to make your first soap with my FREE guide to making soap?



